Research & Articles
Homeopathic Treatment in Children with ADHD: A Randomized Double-Blind Placebo-Controlled Study
Homeopathic Research: Treatment of Hyperactive Children: Results of a randomized, placebo-controlled double-blind study with crossover, long-term follow-up for 8 years
Allowing for the 16% of study participants who did not achieve the eligibility criteria, together with the 11% of participants who switched to methylphenidate after the study, the success rate for long-term homeopathic treatment is 70-75%. This means that three out of four children with ADHD/ADD who commence homeopathic treatment will experience consistent improvement.
INTRODUCTION:
ADHD/ADD is a combination of various cognitive disturbances with hyperactivity/impulsiveness or passivity. With a prevalence of 3-5%, it is one of the most common childhood disorders. Conventional medical treatment consists of stimulants which in most countries fall under controlled substances legislation. Parents who reject such medication for their children are increasingly looking to homeopathy for an answer. Up to the year 2005 there have been two studies on the efficacy of homeopathy for the treatment of ADHD/ADD. Lamont observed significant improvement in outcomes with a simple blind, placebo-controlled crossover study with 43 ADHD/ADD children. In a further study with open clinical observation of progress, Frei and Thurneysen found an improvement in the Conners’ Global Index (see below) of 55% in 86 of 115 children (75%). Since neither study was fully blinded, they could not be regarded as scientific evidence of the efficacy of homeopathy. Two large meta-analyses came to the conclusion that the effect of homeopathy cannot solely be interpreted in terms of the placebo effect. They criticized the unsatisfactory methodology of the studies analyzed and suggested that better-quality studies on particular illnesses should be conducted, which led to the planning of the Bern ADHD/ADD double-blind study. This was conducted with a multidisciplinary study team including the Institute for Complementary Medicine of the University of Berne, the department of child neurology and neuropsychology at the pediatric university hospitals, the Institute for Mathematical Statistics and Actuarial Studies – referred to as IMSV below – and the author’s pediatric homeopathic practice. The design and results of this study are presented below.
DESIGN:
Due to the particularly difficult process of finding a homeopathic remedy for patients with ADHD/ADD, it is only possible to blind such a study after the individual remedy has been found. In the preceding work the authors observed that, when treatment with a potencies is interrupted at an early stage, 49 a worsening of the symptoms occurs within four weeks and that a resumption of treatment leads once more to improvement. This worsening can be used to investigate the difference between a placebo and verum (the active treatment). In the screening phase of the study, all children are initially treated with an open, individualized homeopathic treatment. Those achieving a predefined improvement can then participate in the randomized, placebo-controlled double-blind crossover study, which examines two groups of children in parallel: group A receives verum for six weeks followed by the placebo for six weeks; group B receives placebo for six weeks followed by verum for six weeks; finally, both groups are given verum for six more weeks (group A=VPV, group B=PVV). There then followed an open homeopathic long-term treatment of unspecified duration.
For the screening phase children of both genders between the ages of 6 and 16 were recruited. The children had all been confirmed with a diagnosis of ADHD/ADD according to the DSM-IV criteria36 in a rigorous neurological and neuropsychological examination. It had to be demonstrated that they required treatment of their symptoms and they were not admitted to the trial if they had other chronic illness.
In the crossover study patients were included who achieved an improvement on the Conners’ Global Index of at least 50% or 9 points during homeopathic treatment in the screening phase.
Screening phase: The individual homeopathic treatment proceeded according to the guidelines of Hahnemann and Boenninghausen (including polarity analysis) with Q potencies in daily doses. The precise therapeutic procedure in this study has been described in earlier publications. Any other treatment was either stopped immediately or phased out, and check-ups were made every four weeks. If the reaction was insufficient, the homeopathic prescription was changed.
Crossover study: On admission to the study, the patients were referred back to the university pediatric clinic. At this point they were randomly assigned to group A or B. The patients, their parents, the doctor and the investigator were all completely blind as to the treatment, and the doctor had no contact with the parents and patients during the crossover phase. Children with acute illness, serious accidents or disturbing social experiences were, according to the intention to treat principle, not simply excluded from the final analysis but were included in the evaluation as far as possible.
Long-term progress: After the second crossover period, all patients received an unblinded verum treatment. Further follow-ups occurred at 14 weeks after the crossover period with a questionnaire for the parents and teachers and a written and phone inquiry five and eight years after the start of therapy, conducted by doctoral stu-dents. At this time, the parents were allowed to freely choose their child’s treatment. These final inquiries registered the child’s current treatment and intensity of their ADHD/ADD symptoms.
Measurement (Outcome): The primary outcome variable was the parental rating with the Conners’ Global Index (CGI, table 64). The CGI, a questionnaire of change of behavior (QB), as well as the sub-tests HAWIK-111, K-ABC, VLMT and TAP were evaluated at the start of the screening phase, before and after every crossover period, and six weeks after crossover period 2. To minimize learning effects, only a few of these investigations were identical with those used to make the diagnosis. Fourteen weeks after the crossover study, parents and teachers filled out the Conners’ Parent/Teacher Rating Scales (CP/TRS), and at the five- and eight-year check-ups they evaluated the CGI and indicated their child’s current treatment.
Randomization, blinding, and statistical evaluation: The IMSV generated a randomized list and sealed the assignments to group A or group B in sequentially numbered envelopes. These were handed to the remedy manufacturer, Spagyros, which produced the homeopathic remedies and the placebos. When a child reached the eligibility criteria for the screening phase, Spagyros was informed in writing.
The participating families were then sent the specific medicine (verum or placebo) for the current treatment phase at the begin of each crossover period.

Placebos consisted of 20% alcohol (diluted 1:1000 for use, as was the verum), and were indistinguishable from verum in terms of packaging, labeling, color, smell, and taste. There was no communication between Spagyros on the one hand and the study participants, the doctors and the psychologists on the other – except if a child had to leave the screening phase due to unexpected difficulties. The statistical evaluation by the IMS was also blinded – the statisticians did not know to which group the patients were assigned to.
RESULTS:
Of 140 children recruited, 83 met the initial criteria and were admitted to the screening phase. The average CGI rating at the start was 19, with a range 15-25. Seventy children (84%) met the eligibility criteria for the crossover study and 62 children in fact took part in the crossover study. The following data originate only from the 62 children in the crossover study.
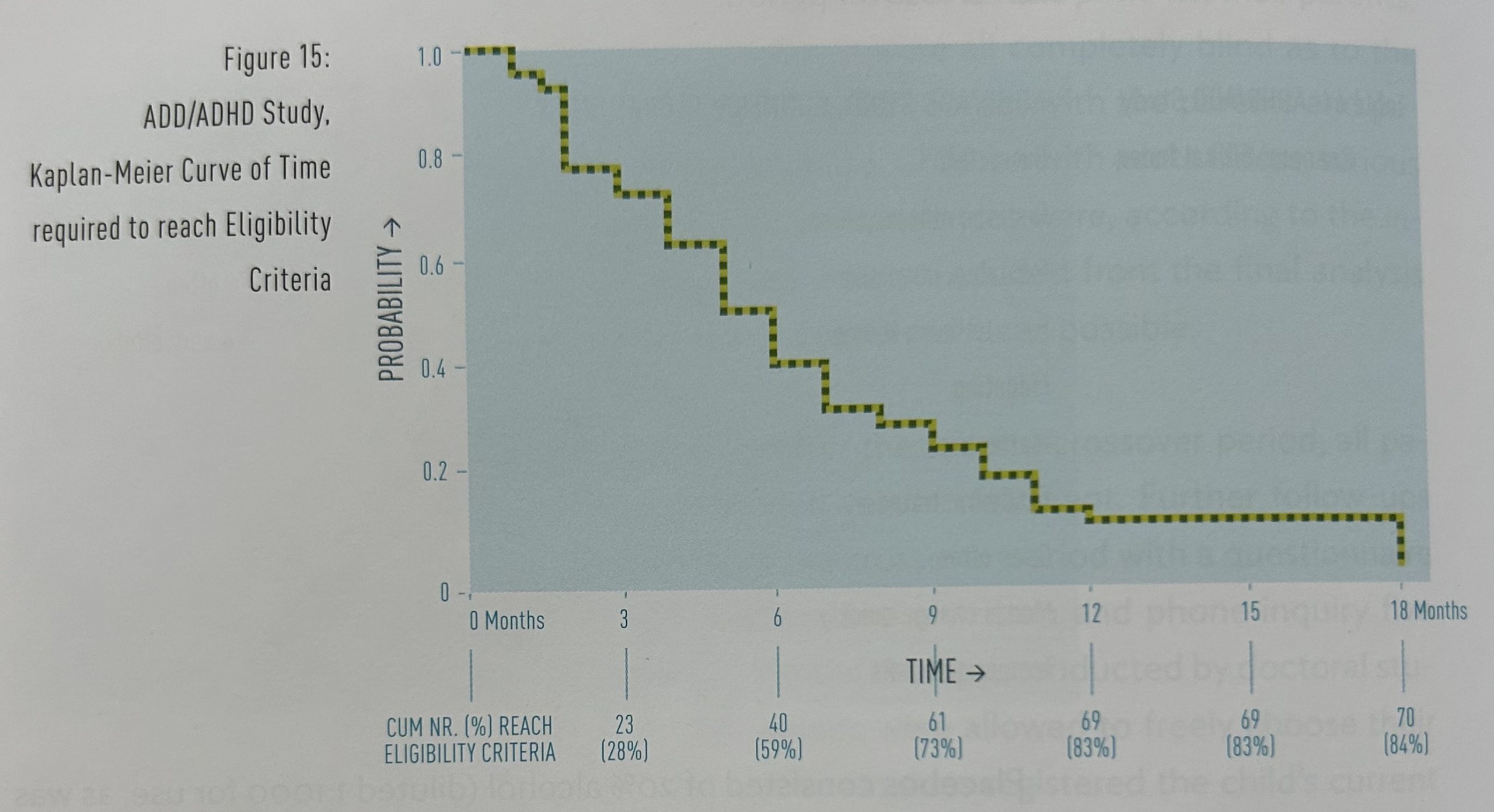
Time needed to reach the eligibility criteria: The patients reached the eligibility criteria for the crossover study after an average treatment period of 5.1 months (standard deviation 3.20, range 1-18 months), with an average CGI rating of 8 (range 4-15). Figure 15 shows the treatment period required by individual patients to reach the eligibility criteria.
The 62 patients were successfully treated with 17 different homeopathic remedies (table 65). Remedies were classified as successful if the patient met the eligibility criteria for the test phase. Further remedies were used but did not lead to a sustained improvement at the required level; they are therefore not included.

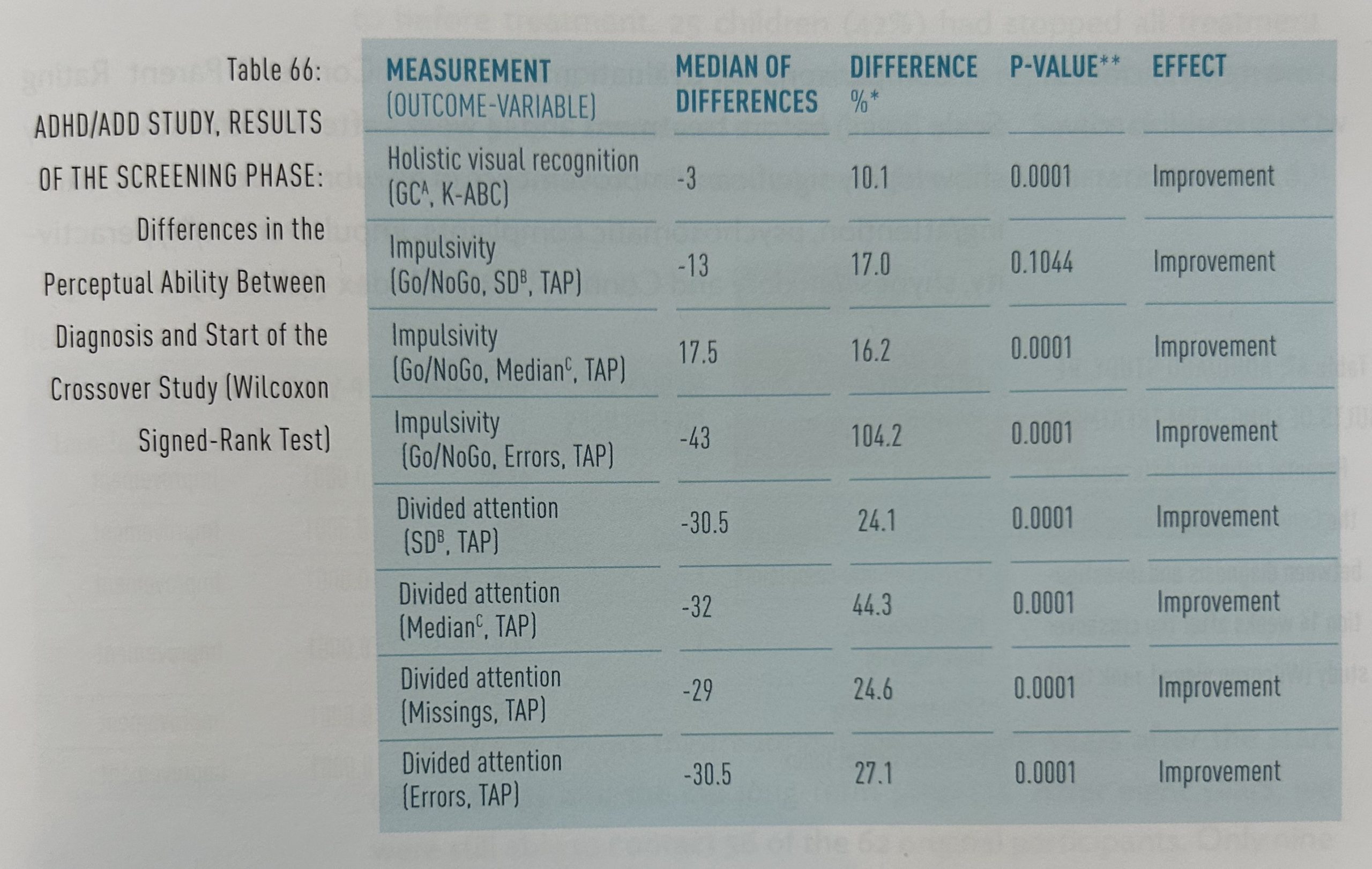
Some neuropsychological investigations were conducted, both on diagnosis and on entry to the crossover study, enabling a comparison of the untreated state with the state following homeopathic treatment. Highly significant improvements were seen in the ability to recognize visual details, attention capacity and impulsivity (table 66).
Out of 62 patients, three dropped out of the study during the first crossover period and one during the second crossover period. The reasons for dropping out were increasing tics, behavioral disturbances and a reactive depression. These patients were included in the final analysis according to the intention-to-treat principle.
The comparison of the treatment effect – “within-patient differences” – shows that the CGI with verum compared to placebo declines on average by 1.67 points. This improvement is statistically significant with a p-value of 0.0479 and a 95% confidence interval (CI) of -3.316 – 0.016. Further neuropsychological investigations during the crossover study produced a significantly improved resistance to verbal interference (VLMT test), with a p-value of 0.0328, a trend towards stabilization of mood (p=0.0693) and a trend towards improvement in reaction to unpredicted events (p=0.1001).
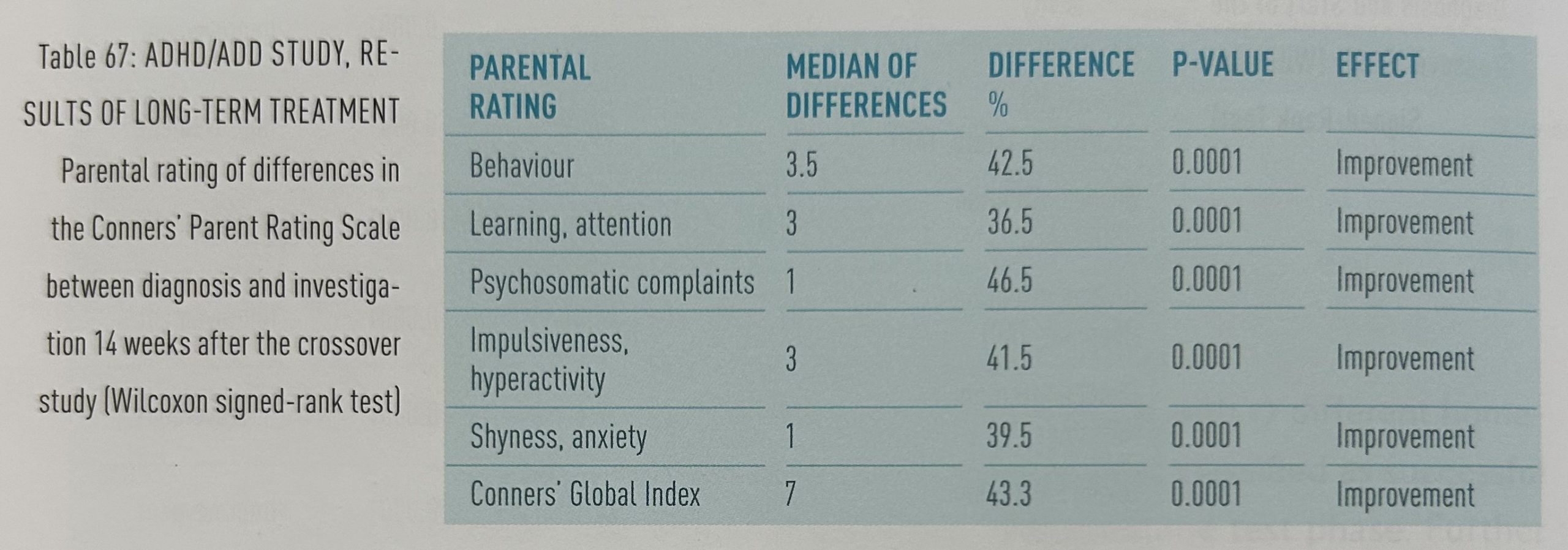
Comparisons of evaluations with the Conners’ Parent Rating Scale (CPRs) before treatment and 14 weeks after the crossover study show highly significant improvements in all rubrics: behavior, learning/attention, psychosomatic complaints, impulsiveness/ hyperactivity, shyness/anxiety and Conners’ Global Index (table 67).
The ratings with the Conners’ Teacher Rating Scale (CRS) also showed a significant improvement in behavior, as well as trends towards improvement in learning and attention, passivity and the Conners’ Global Index (table 68).
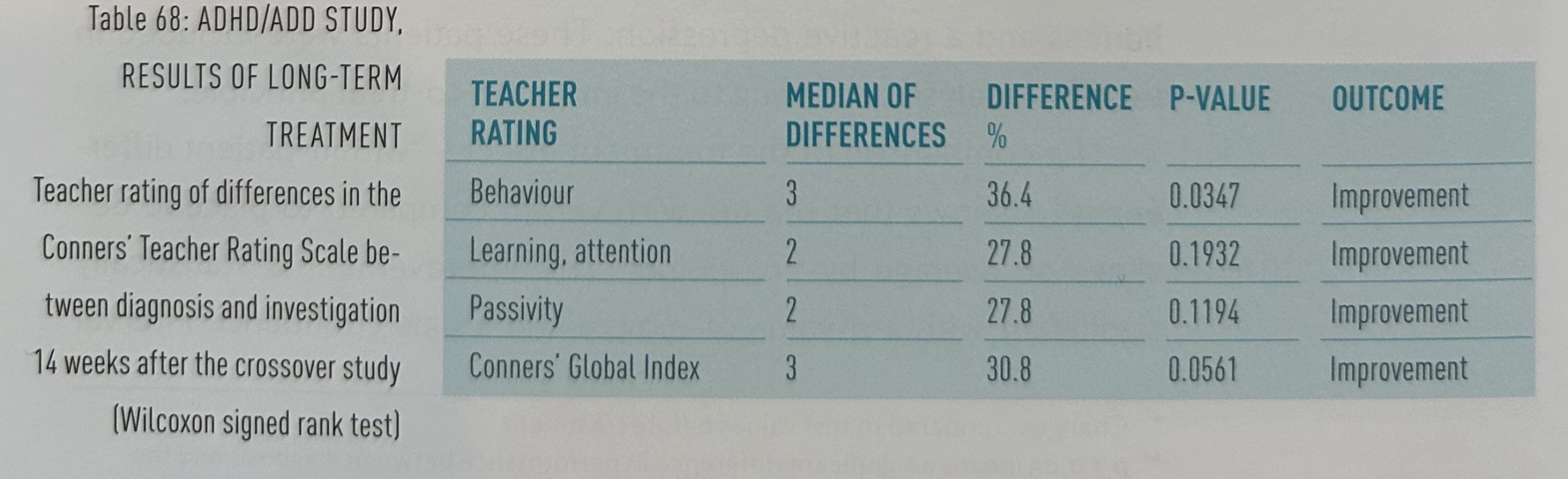
Figure 16 shows the therapy status five years after the start of the study as well as the CGI ratings from parents at this time. From 14 weeks after the end of the study, the patients and their parents were free to choose how to continue treatment. At this five-year point in time, we were able to contact 6o of 62 sets of parents whose children took part in the crossover study. Twenty-eight children (47%) were still being treated with homeopathy, with an average CGI rating of 6.8, which corresponds to an improvement in the CGI of 64.3% compared to before treatment. 25 children (42%) had stopped all treatment. Their CGI rating was 8.8, which nevertheless represents an improvement of 53.5% compared to the initial value. Seven children (11%) were being treated with methylphenidate. Their CGI rating was 10.6.
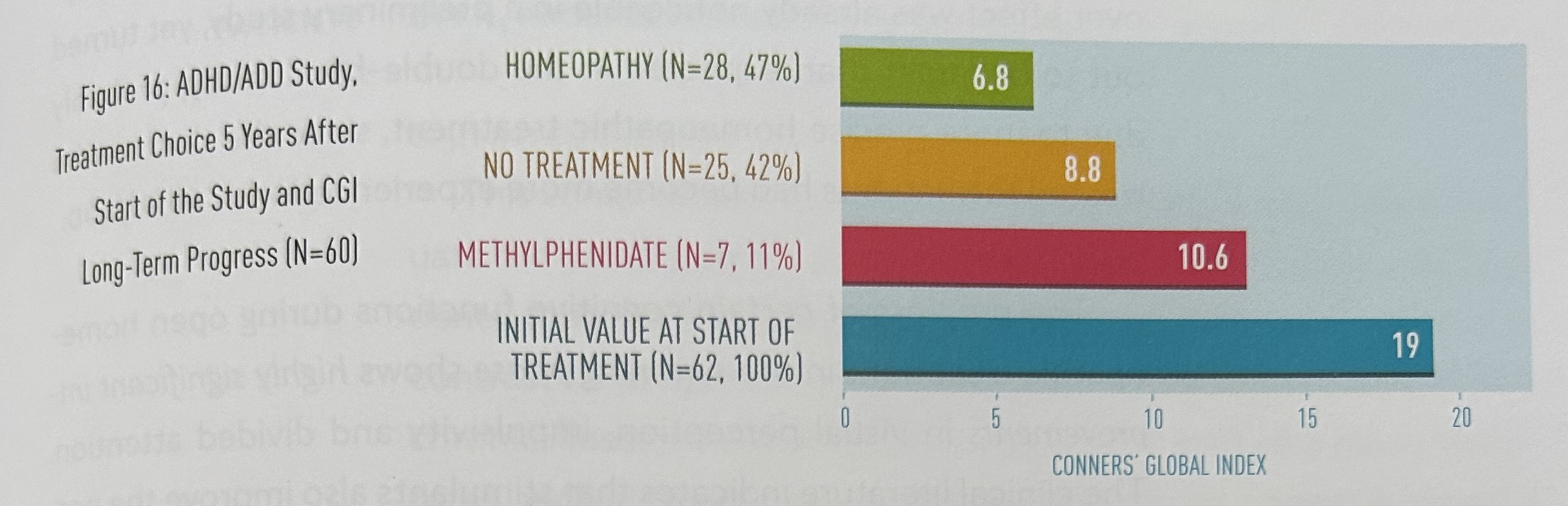
Figure 17 shows the treatment choice eight years after the start of the study and the CGI long-term progress. After eight years, we were still able to contact 56 of the 62 original participants. Only nine patients (16%) were still having homeopathic treatment at this time. Their CGI ratings were unchanged at 6.8. Thirty-eight patients (68%) were not undergoing any form of treatment, and their average CGI rating was 8.8. A further nine patients (16%) were taking methylphenidate: their average CGI rating was 8.0. The difference in CGI ratings between the three groups is not significant.
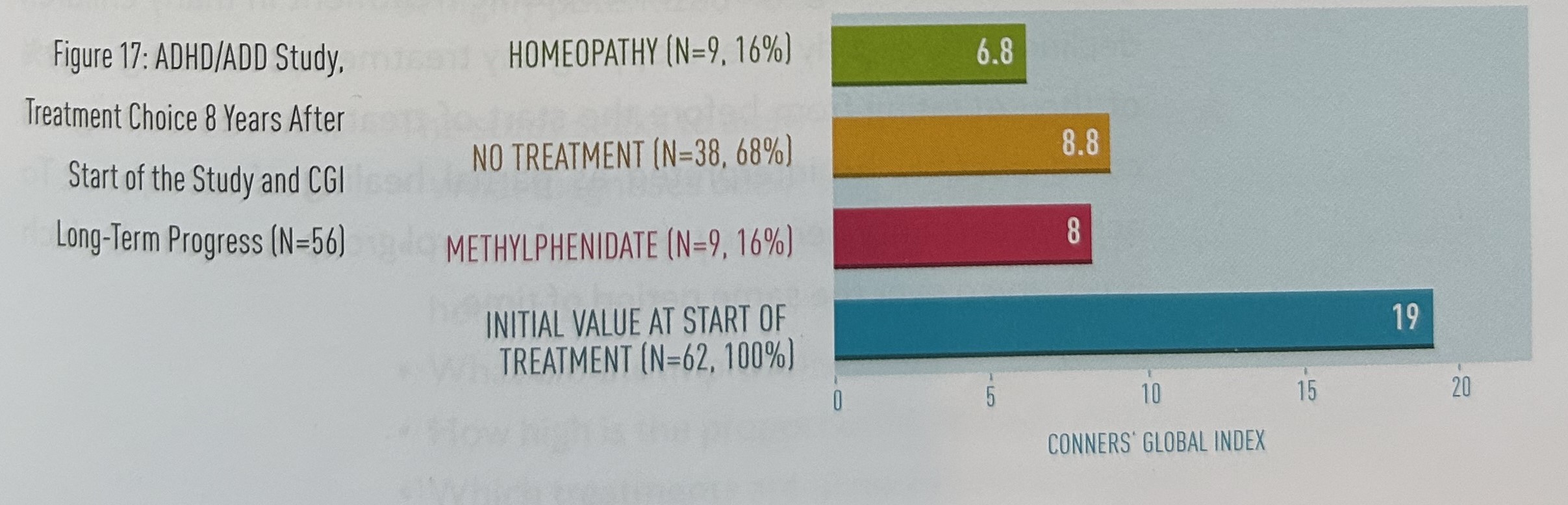
DISCUSSION:
The randomized double-blind study shows a significant effect of homeopathy for children with ADHD/ADD. The chosen design enabled an individual prescription of homeopathic medication, which is the main requirement of homeopathy when correctly practiced. Significant means that the effect of homeopathy, even under the strict scientific conditions of a double-blind study, differs from that of placebo. Due to a very strong carryover effect, the CGI difference of 1.67 between placebo and verum was smaller than expected. The carryover effect was already noticeable in a preliminary study, yet turned out to be larger than expected in the double-blind study, probably due to more precise homeopathic treatment, since the doctor who treated the patients had become more experienced with ADHD/ADD.
The progress of certain cognitive functions during open homeopathic treatment in the screening phase shows highly significant improvements in visual perception, impulsivity and divided attention.
The clinical literature indicates that stimulants also improve the perception deficits found in ADHD/ADD patients. In order to investigate quantitative aspects more precisely, a study with comparable patients using homeopathy, stimulants and placebo would be valuable.
The long-term progress shows that the CGI and CPRs values (parental assessment) also showed a highly significant decline of 37% to 64%. This means that the intensity of the ADHD/ADD symptoms declined, accompanied by clear improvements in the emotional and social aspects as well as better behavior at school. It was surprising that the five-year check-up indicated that the improvement achieved as the result of long-term homeopathic treatment in many children declines only slightly after stopping any treatment, stabilizing at 47% of the CGI rating from before the start of treatment. Such progress could possibly be interpreted as partial healing of ADHD/ADD. To achieve certainty here, an untreated control group is required, which is observed over the same period of time.
CONCLUSIONS:
Allowing for the 16% of study participants who did not achieve the eligibility criteria, together with the 11% of participants who switched to methylphenidate after the study, the success rate for long-term homeopathic treatment is 70-75%. This means that three out of four children with ADHD/ADD who commence homeopathic treatment will experience consistent improvement.
Source: heinerfrei.ch/publications
Frank Molignano, CCH, RSHom(NA), is a board-certified homeopath, educator, and clinic supervisor with over 15 years of experience helping individuals manage ADHD, mental health challenges, trauma, and chronic conditions. He works with clients both in-person and virtually, making homeopathy accessible no matter where you are. When he’s not helping clients reclaim their focus and balance, he enjoys bouldering, paddle-boarding, and dancing. Learn more about Frank here.